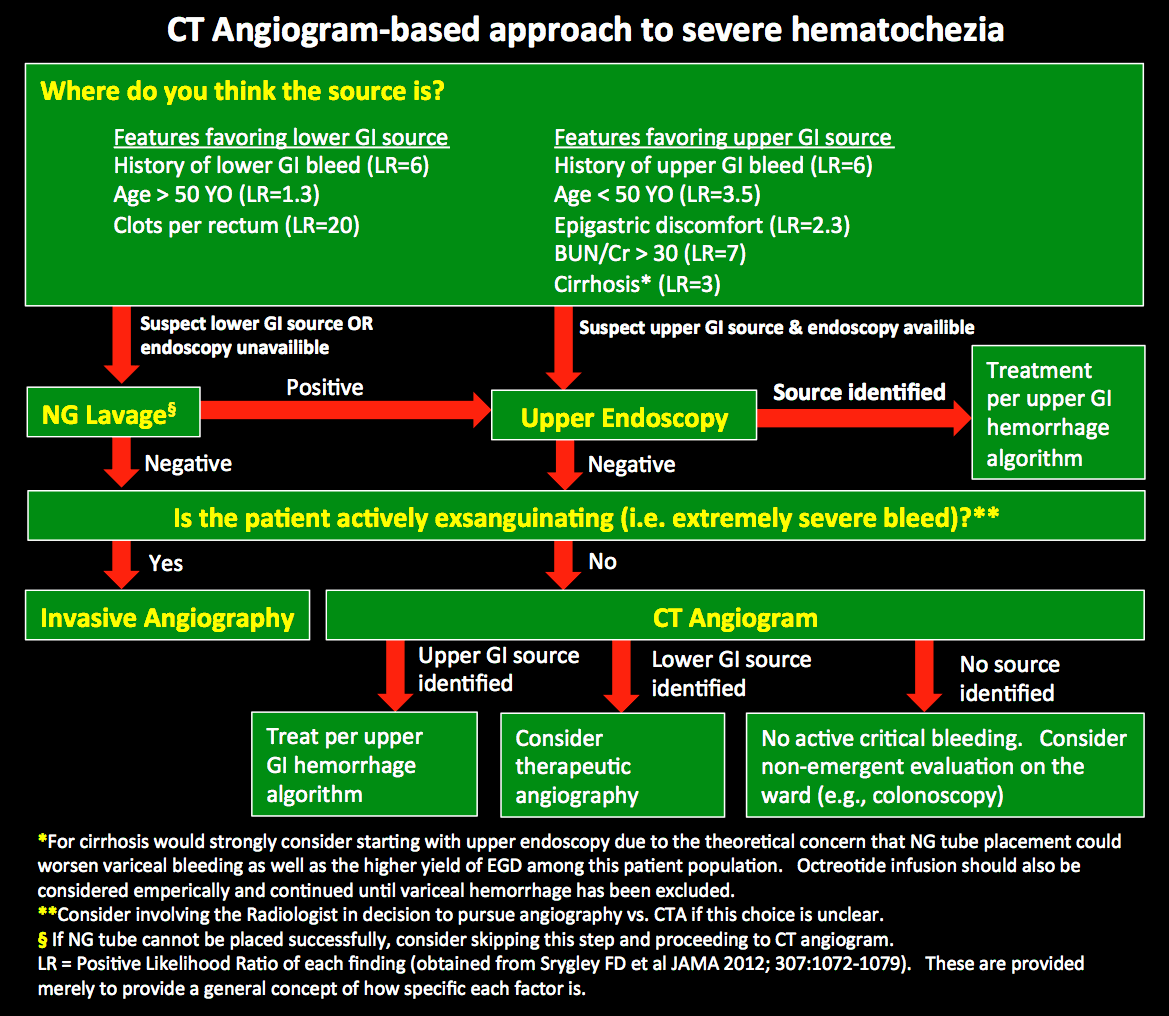
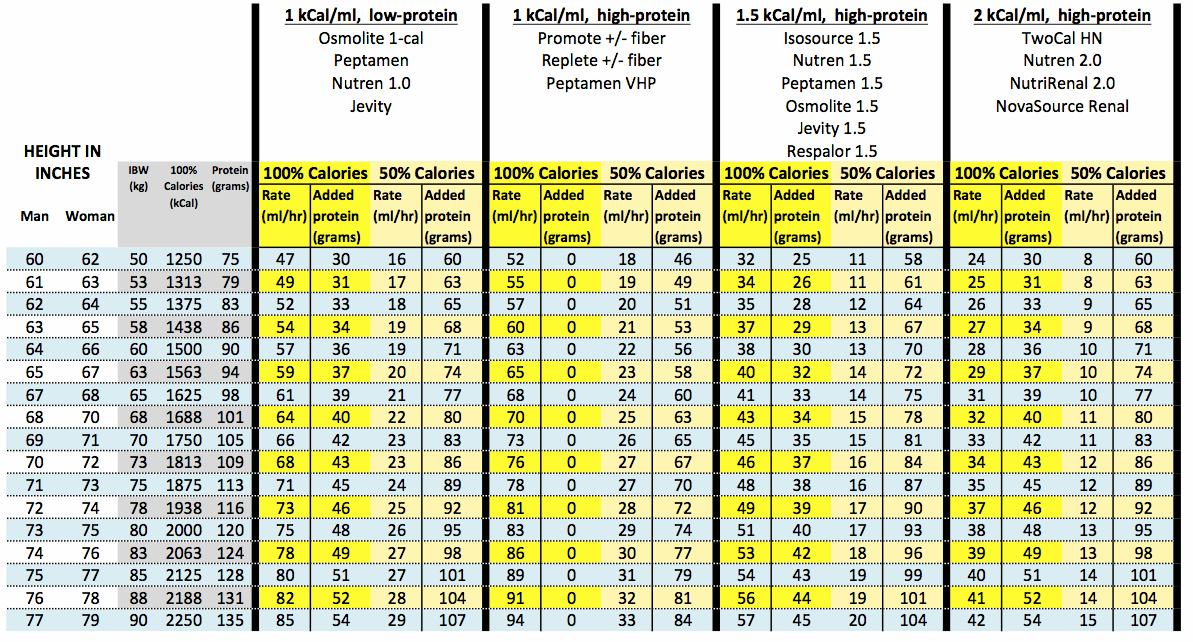
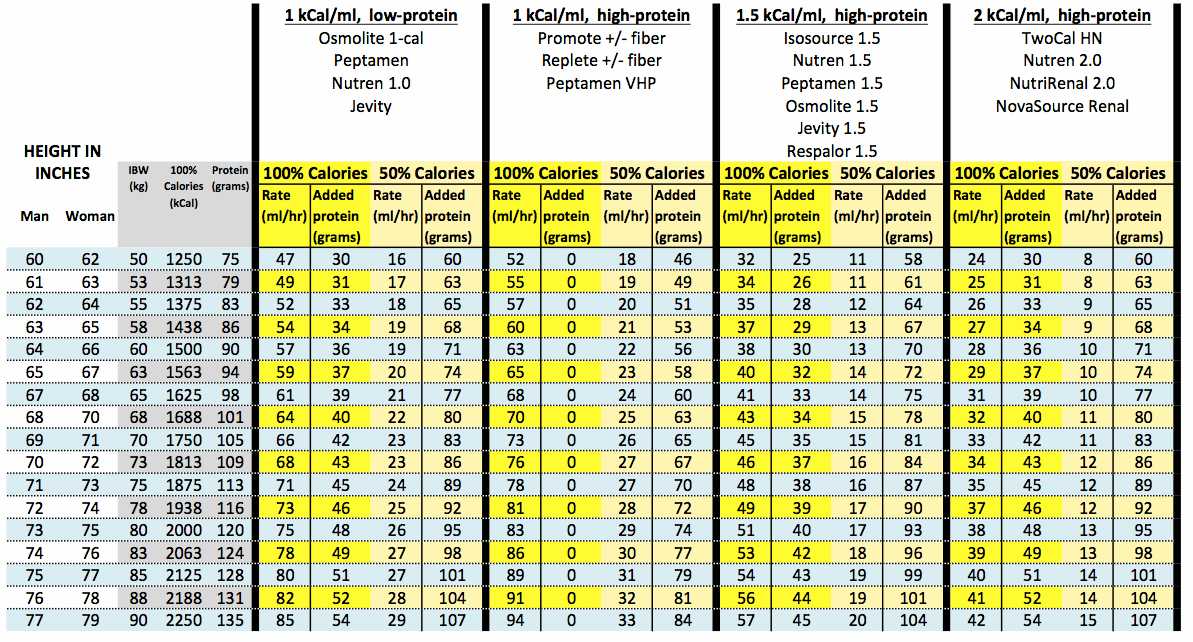
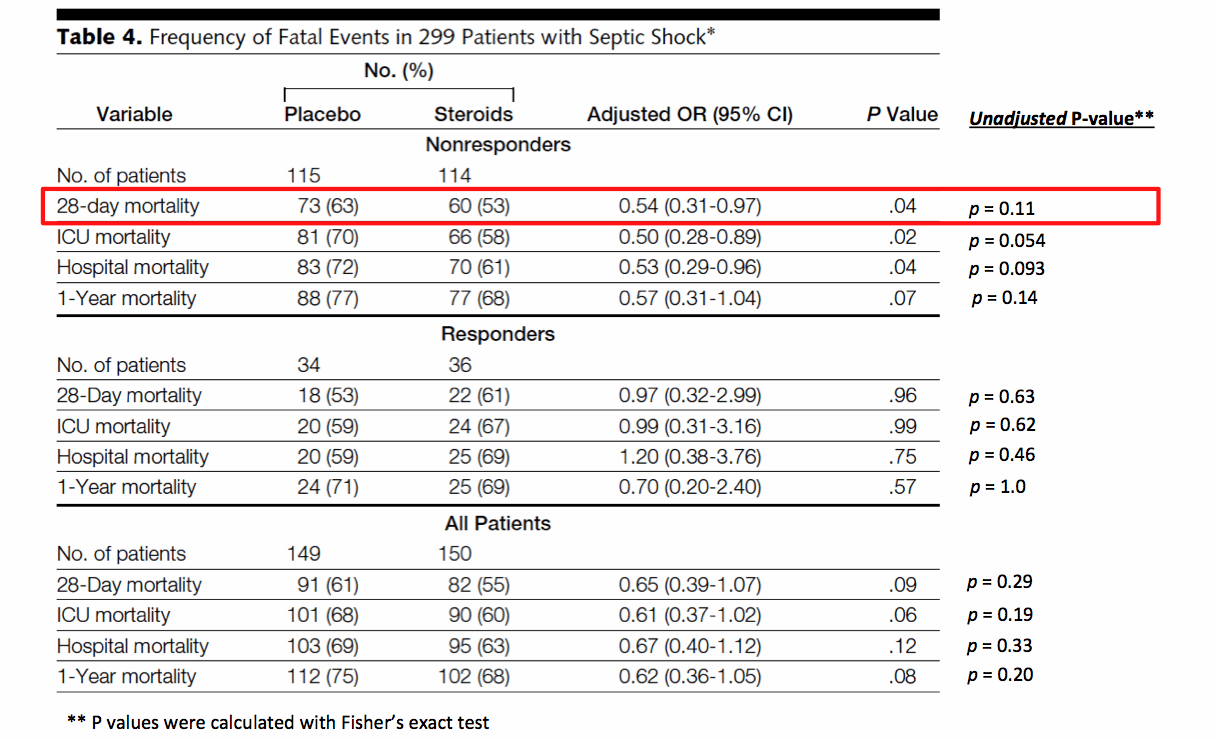
Introduction
Recently the New England Journal of Medicine launched a media campaign challenging the negative perception of industry conflicts of interests (COI). This was surprising, because it is the opposite of what editors of the NEJM have previously reported (see above books by former NEJM editors, published in 2004 and 2005). Big pharma hasn't reformed dramatically in the last decade. So why the change of heart?
History of the NEJM & COI
Some context is helpful. In 1996, the NEJM editors Drs. Angell and Kassirer made it official policy of the journal that reviews and editorials could never be written by authors with financial COIs (Angell 1996). However, these editors both left the NEJM, possibly related to a disagreement with their publisher's plans to use the NEJM's brand to promote other sources of healthcare information (Smith 2006). Subsequently, Dr. Drazen was appointed as editor-in-chief of the NEJM in 2000, despite concern that he had ties to numerous drug companies (Sibbald 2000, Gottlieb 2000). In 2002 the NEJM changed its policy toward COIs, allowing editorials and reviews to be written by authors with "insignificant" financial COIs (defined as <$10,000 per year from industry)(Drazen 2002).
This policy reversal is best described by Dr. Kassirer:
"During the decade of the 1990s, when I was editor-in-chief of the New England Journal of Medicine, we rejected anyone who had a conflict of interest from writing an editorial or review article. Sometimes it required going down the list until we found someone who didn't have a conflict, but we never had to compromise and accept someone without sufficient expertise to do a good job. I also think it's often a good idea to get someone who isn't too close to the action: it often avoids "group think" and provides a fresh perspective. But to maintain our 1990s policy takes more work because you can't just accept the first person who pops into your mind. I was disappointed when the journal changed the policy, and said so publicly."
- Kassirer JP, British Medical Journal 2001
The current media campaign is a continuation of the direction that the NEJM set forth in 2002. Perhaps the campaign represents a response to the British Medical Journal, which recently announced a new "zero tolerance" policy in which no financial COIs will be allowed for authors of editorials or reviews (Chew 2014).
Current media campaign in the NEJM
This has consisted of a three-part series of articles by Dr. Rosenbaum, an editorialby NEJM editor-in-chief Dr. Drazen, and a reader poll. The overall message is that we have grown overly suspicious of big pharma and COIs. "We have forgotten that industry and physicians often share a mission - to fight disease."
Although Dr. Rosenbaum's articles make some valid points, they are quite one-sided. Dr. Rosenbaum is a correspondent for the NEJM, so it is no coincidence that her articles are strongly supportive of the current policy initiatives of the NEJM. Ironically, this exemplifies the significance of COIs. Although it is impossible to know how much her position may have affected her perspective, her COI naturally challenges her unbiased opinion on the matter.
Perhaps the most interesting component of the media campaign is the reader poll about the adequacy of various hypothetical authors for a review article. Three potential authors are described, all of whom have significant COIs. The design of this poll itself is biased, by presenting no authors without COIs. A more transparent approach might be to simply ask readers "do you think review article authors should be allowed to have COIs?"
Industry funding of NEJM
The NEJM itself has significant financial conflicts of interest. This may not come primarily from print and electronic advertisements by drug companies, but rather from industry purchases of article reprints. For example, if the NEJM publishes an article supporting a new drug, the drug company will often purchase thousands of reprints of the article. The NEJM makes a large profit margin from the reprints. For example, Merck purchased 929,400 reprints of the infamous VIGOR trial of Vioxx, yielding an estimated income for the NEJM of $697,000 (Marcovitch 2012). The Lancet editor Dr. Richard Horton reported that companies may promise to purchase a large order of reprints in return for publication of a favorable study.
It is impossible to determine how much money the NEJM makes from reprints. Although the BMJ and Lancet disclosed their income from reprints, the NEJM and JAMA have not done so (table above). Between 2005-2006, the sale of reprints contributed 41% of the total income of the Lancet. The NEJM likely receives more revenue than the Lancet from reprints, given that it publishes more industry-supported studies than the Lancet (table below). Combining revenue from advertising and reprints, it is likely that the NEJM receives most of its revenue from industry.
In 2008, Dr. Drazen favorably reported the revenue from industry in a meeting of the Massachusetts Medical Society (publisher of the NEJM), saying "The results in recruitment advertising and bulk reprints were outstanding this year; They went a long way to offset declines in print-based revenue that all publishers are experiencing." (BMJ 2011).
Conflicted nature of medical publishing
Like drug companies, medical journals have a conflicted set of incentives (Marcovitch 2010). Certainly, any journal has lofty philosophical goals such as improving medical care. However, the journal is also a news organization, and as such may be drawn to the hottest news stories. Finally, any journal functions within a business model, requiring sufficient revenue to stay solvent.
These incentives may be conflicting. For example, journals often tout their impact factor, a measurement of how well they are read and cited. Less publicized is the frequency of article retractions. Compared to other journals, the NEJM has both the highest impact factor and also the highest frequency of retractions (Fang 2011). This suggests that in the pursuit of hot articles, corners are sometimes cut.
Managing COI: Who should write review articles and guidelines?
There are two general concepts for approaching the authorship of NEJM review articles (and guidelines in general). The traditional approach is the subject matter expert model (below). In this model, a handful of experts are involved in performing industry-funded research. These experts, who usually develop some COIs, are also involved in authorship of guidelines and NEJM review articles. This is the model which the NEJM is currently promoting. For example, in his editorial Dr. Drazen reflected on the virtues of a simpler time in the 1940s when a single investigator could discover and market streptomycin, and then write a major review article on the same topic.
A newer approach might be described as a COI-free model (above), wherein guidelines and NEJM review articles are authored by experts without COIs. Since investigators are often involved in industry-funded research and frequently have COIs, this would mean that prominent investigators would often be excluded from authoring guidelines and review articles in the NEJM. As discussed above, this approach requires more work because qualified experts without COIs must be sought. However, unbiased experts will provide fresh perspectives which add diversity to the field.
A recent example of these two models was the evolution of the American College of Emergency Physicians (ACEP) clinical policy regarding ischemic stroke. Initially, a policy was drafted as a joint document with the American Academy of Neurology, including authors with COIs. The first version was very enthusiastic about the use of TPA (giving it a Level A recommendation within the 0-3 hour window). This policy, and concerns about COIs, caused an uproar. ACEP consequently broke away from the American Academy of Neurology and went back to the drawing board to design an entirely new policy authored solely by experts without any COIs. The new policy is generally felt to be a major improvement compared to the initial policy, with less bias and more focus on the evidence.
Is there a shortage of authors for review articles?
The argument for allowing authors with COIs to write NEJM review articles is based on a reported shortage of eligible authors (as described in the 2002 NEJM policy statement here). This is hard to believe. For example, in the USA alone there are >150,000 active full-time faculty employed by medical schools. Any of these faculty would probably be honored to write a review article for the NEJM, and many thousands of them are qualified.
Conclusions
The recent NEJM campaign in support of industry is partially correct: COIs are not necessarily evil, and people with COIs include many brilliant researchers and clinicians. Certainly physicians and pharma need to work together to develop new drugs, with patients often benefitting from such collaboration.
However, there is no shortage of unbiased experts without COIs to write NEJM review articles and consensus guidelines. Choosing physicians without COIs for these tasks makes sense. This would avoid bias or the appearance of bias, thus bolstering trust in these sources. As a clinician, I would be more interested in a review by an author without COI.
Evaluating this issue exposes the fact that medical journals have significant COIs. Journals often receive significant funds from drug companies in direct response to publishing industry-funded research (in the form of bulk reprints). With the British Medical Journal and the NEJM moving in opposite directions on this issue, further examination of these differences is necessary.
Additional reading
- No, Phramascolds are not worse than the pervasive conflicts of interest they criticize: Larry Husten in Forbes
- Medical journals are an extension of the marketing arm of pharmaceutical companies. Smith R, PLOS Medicine 2005.
Conflicts of Interest: None.
Image Credits: Image of physician obtained from http://www.cliparthut.com/doctor-symbol-clipart.html